Biodegradation of implant materials and medical product components
From a technological and regulatory perspective, innovative implants are among the most challenging medical products. Therefore, when it comes to selecting the implant material and the materials for medical product components, the critical minimum requirements must be analyzed first. The materials’ biodegradation is an important factor in this.
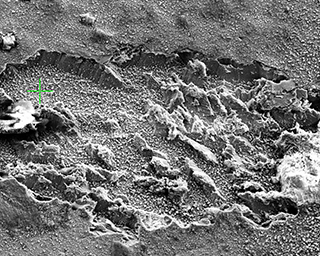
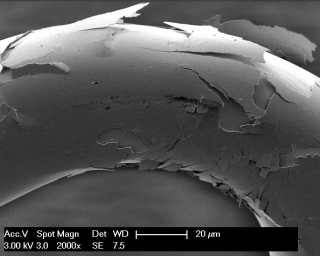
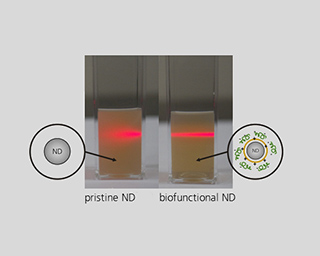
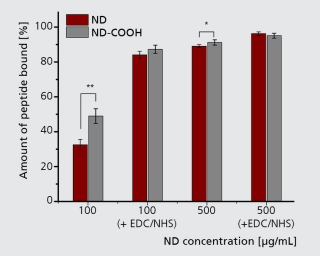
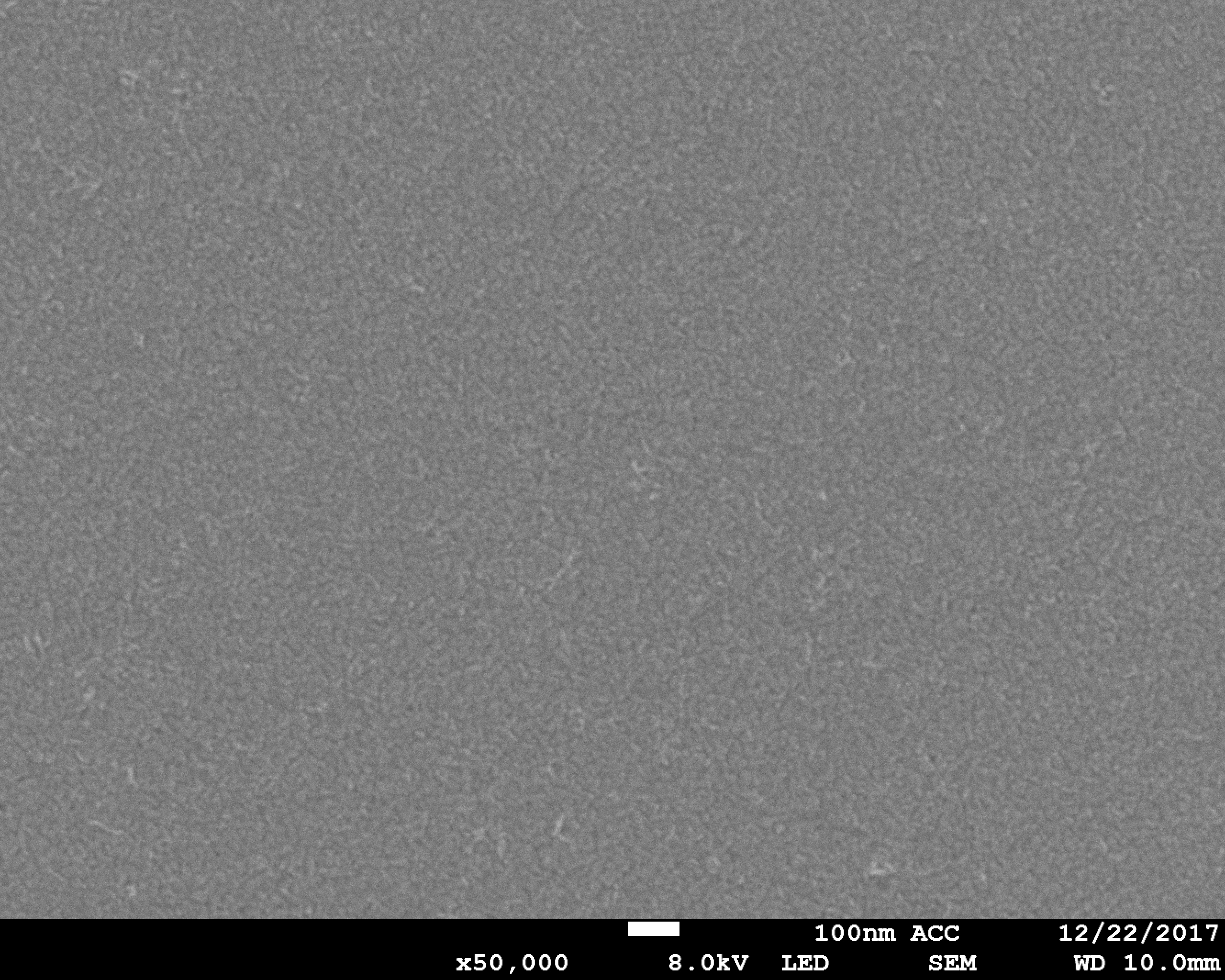
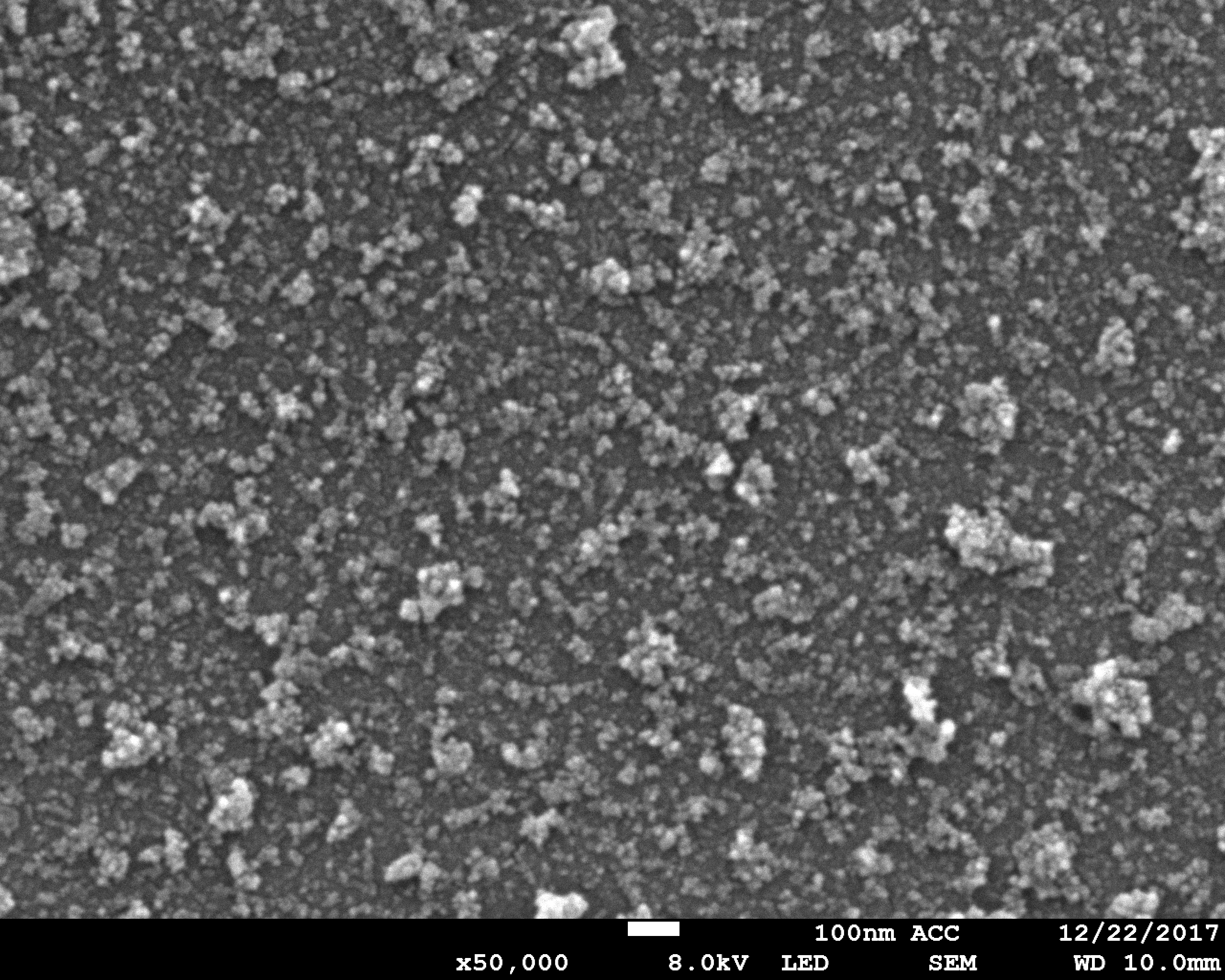
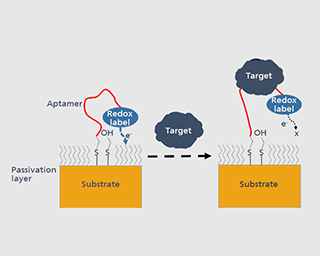
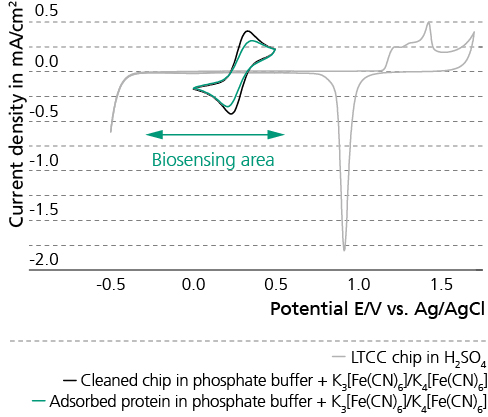
Tissue and body fluids come into contact with the implant surfaces. The interactions caused by this fact require detailed in-situ monitoring of the various phases of the degradation process. In the worst case, interactions result in the failure of the implant’s functions, decomposition or unwanted physical responses. Therefore, the Biodegradation and Nanofunctionalization workgroup investigates material changes and aging processes by using synthetic physiological media. The IKTS researchers develop innovative methods to analyze the biodegradation of various material classes and apply these methods.
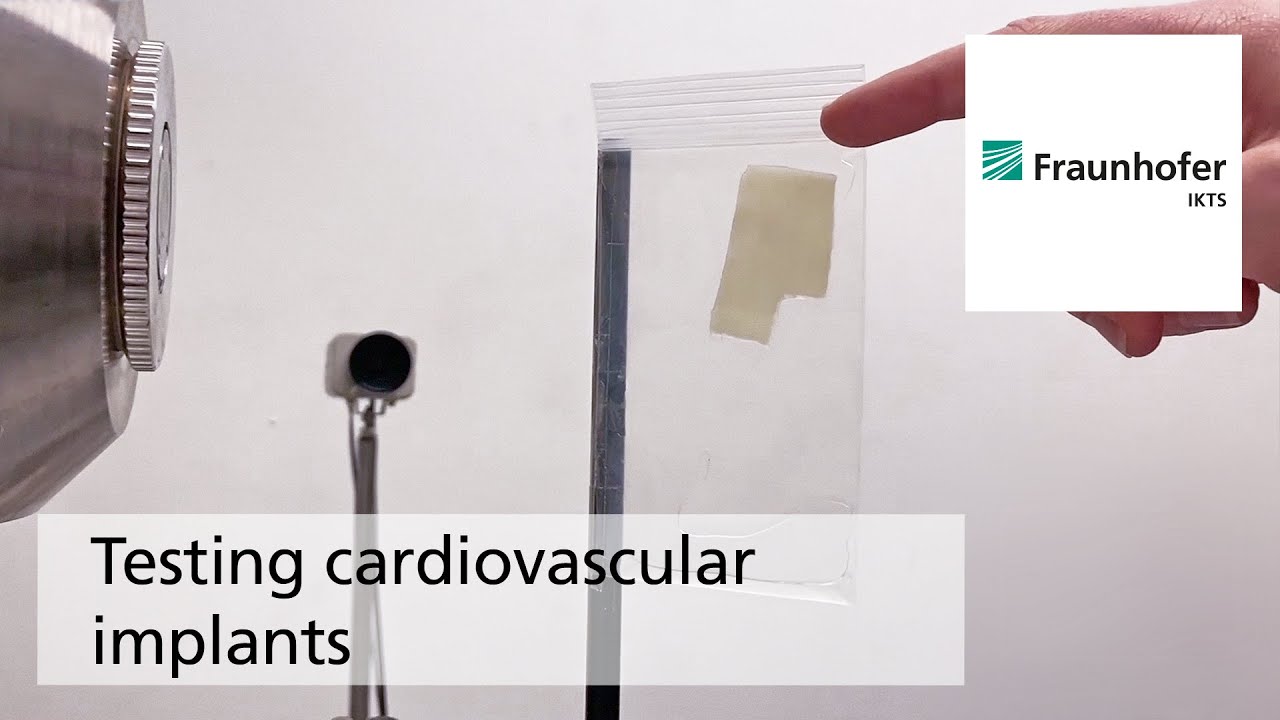
The biodegradation analyses are accompanied by comprehensive characterization of physical-chemical material properties. The research and development efforts contribute to the optimal design of novel bioresorbable implants, such as stents, locating screws and bone substitute material. But optimization also extends to bioresorbable, drug-eluting implant coatings. The workgroup tries to determine the exact local and chronological process of degradation with regard to physiologically related environmental conditions (temperature, medium, mechanic stress). This enables manufacturers to influence the spatial original design and the manufacturing conditions of the degradable material or implant.