
As part of the Department of Stationary Energy Storage, the “Ceramic Electrolytes and Electrodes” group works on new materials and components for energy storage and conversion.
Ceramic electrolytes and active materials for sodium-based battery cell systems
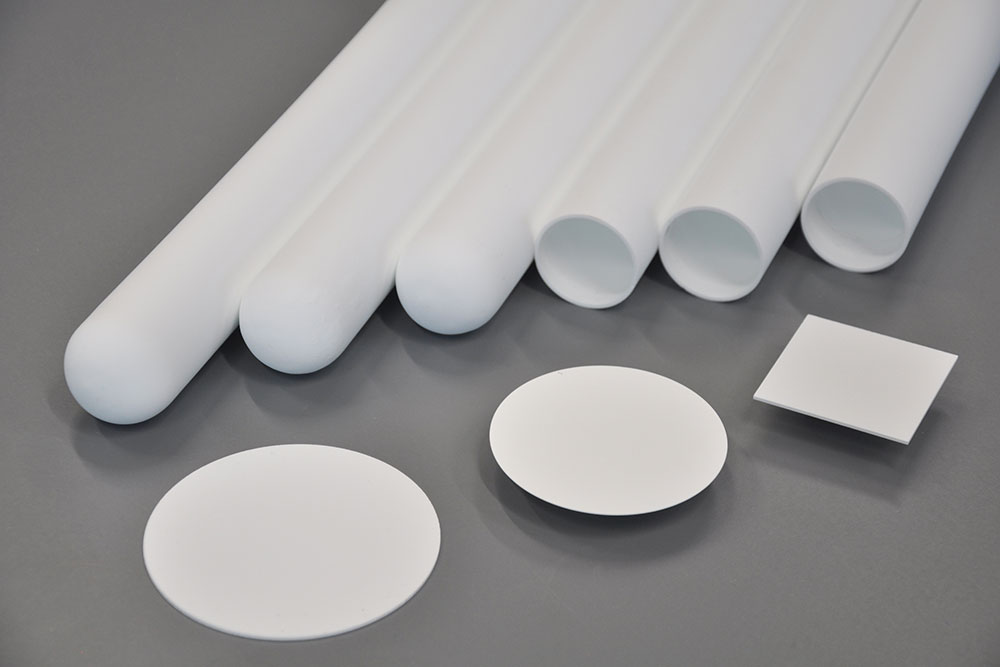
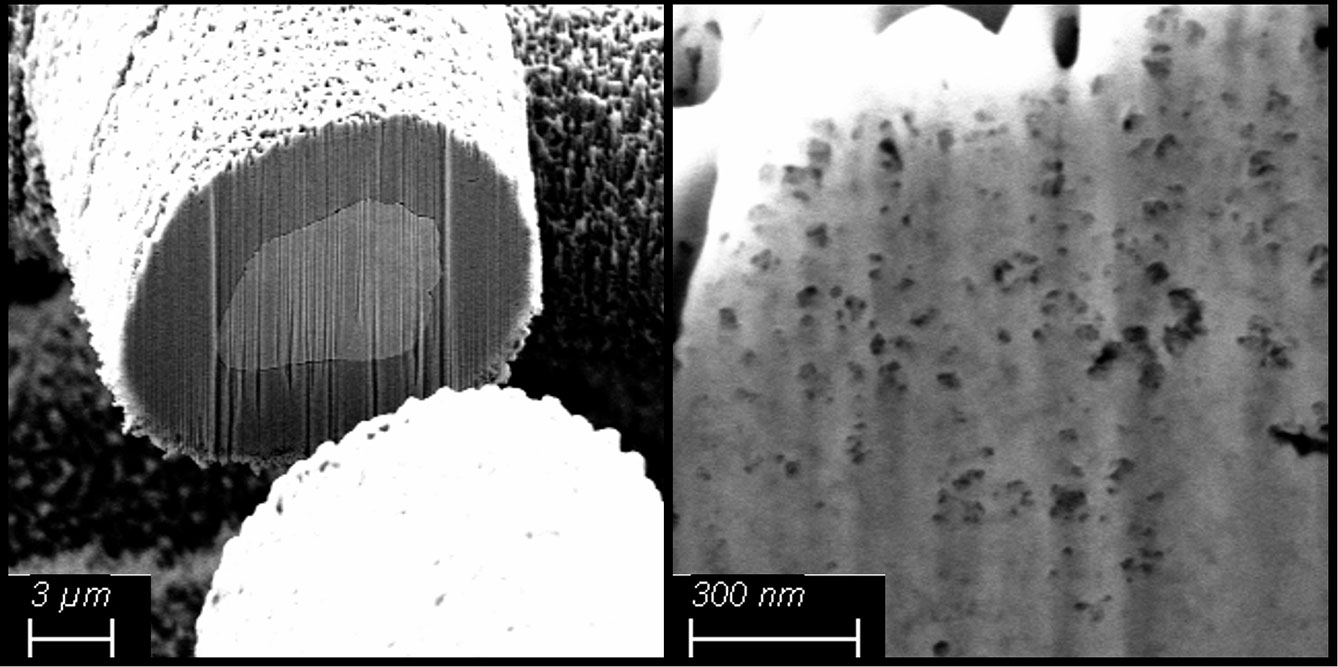
One focus of the group is on ceramic electrolytes based on Na-β"-aluminate. In addition to its high ionic conductivity both at high temperatures and at room temperature, Na-β"-aluminate has the advantage of being stable towards sodium. This makes it possible to use elemental sodium as an anode in cells. Solid electrolytes made from Na-β" aluminate can be produced in both planar and tubular geometries in small to medium quantities on the pilot plants at Fraunhofer IKTS. Manufacturing methods such as uniaxial or isostatic pressing, casting or cold plastic extrusion are available.
In addition to ceramic electrolytes, research is also being conducted into active materials such as LNMO or NVP for sodium-based cell systems. Laboratory-scale synthesis routes and scaling options up to 50 kg are available for this purpose. The synthesized active materials are integrated into electrodes and electrochemically qualified in existing measuring systems.
Electrodes for electrochemical reactors
The interdisciplinary research group also focuses on the development of electrodes with high current density, efficiency and durability – tailored to different electrochemical reactors:
- Alkaline water electrolysis (green hydrogen)
- Electrochemical H2O2 production (Advanced Oxidation Processes, AOP)
- CO2 reduction (formation of hydrocarbon compounds)
- Metal-air batteries (e.g. iron, zinc or Al-air batteries)
Especially in the field of gas diffusion electrodes, Fraunhofer IKTS has extensive experience in material selection, process development and application-oriented optimization.
Key components are electrocatalysts, which we develop using various methods and integrate into electrochemical reactors. On the one hand, we use electrochemical coating of customized electrocatalysts for the respective reaction. This allows surface structures to be modified to achieve high current densities and low losses. On the other hand, corrosion protection coatings can also be realized for the respective problem.