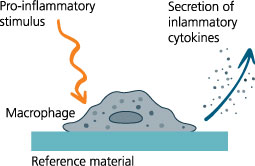
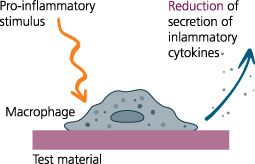
Established testing system (ClicKit-Well) for biological material testing in direct cell-material contact
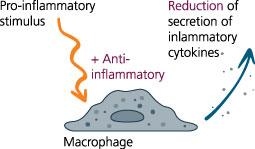
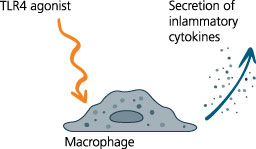
Conventionally, materials are examined in vitro in cell culture plates, which makes quantitative comparisons between materials (test specimens), e.g., with different geometries, difficult.
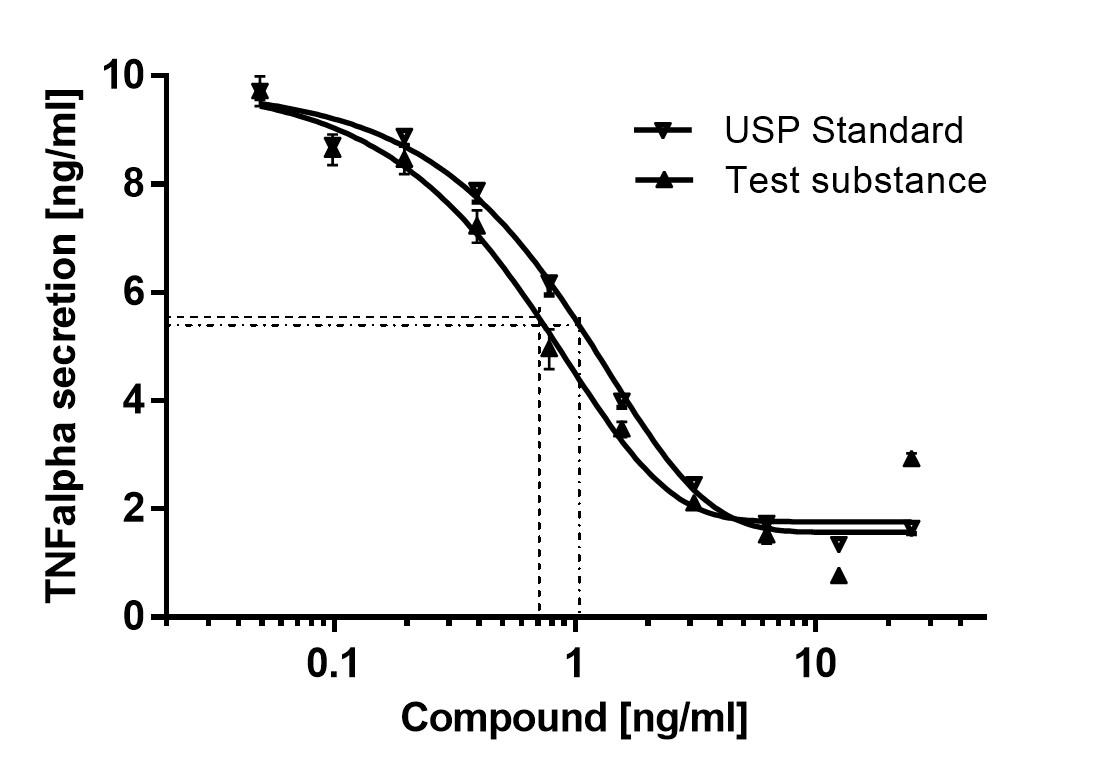
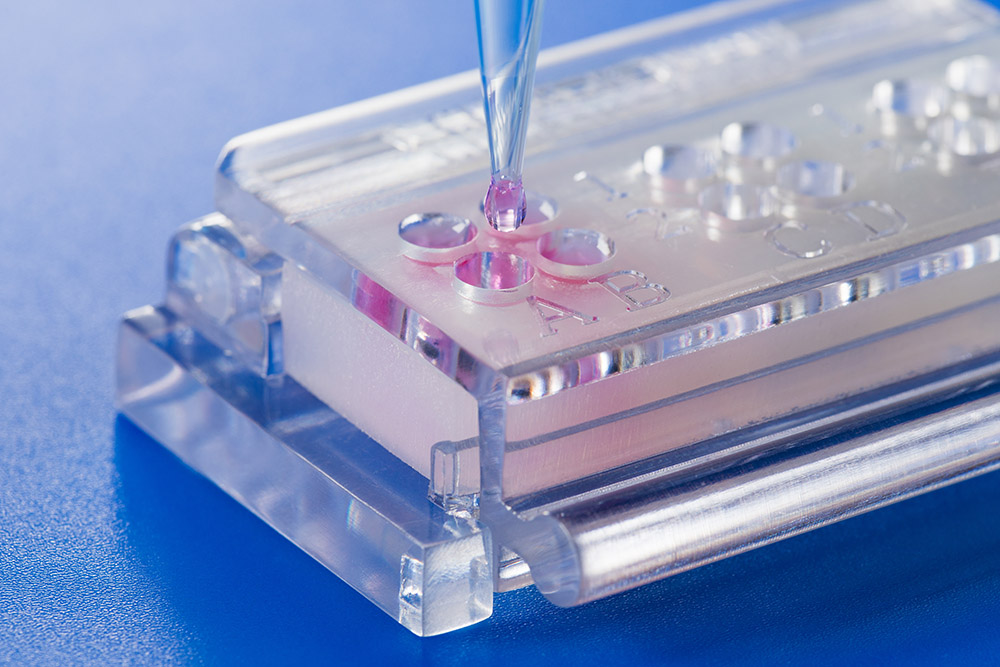
The patented in-vitro test system ClicKit-Well was developed for the reliable analysis of surfaces (DE 102018221415 B3). With its surface standardized areas (e.g., 96-well format) on material test specimens, it allows quantitative comparisons between materials and coatings.
Further information incl. a video showing the working principle can be found at the end of the page at “Biomaterial testing 2.0 – standardized, resource-efficient: ClicKit-Well”.
Topic-adressing projects:
- BMWi – WIPANO project (knowledge and technology transfer through patents and standards) with the aim of developing a quantitative direct cytotoxicity test (as a standard test method) for medical devices – cooperation project with Eurofins BioPharma Product Testing Munich GmbH and DIN
- EU-HORIZON cooperation project with the topic “Next Generation BiOactiVe NAnocoatings“ (project acronym: NOVA)
- VDE/VDI BMBF VIP+ cooperation project with the topic “Universally usable in-vitro testing system for standardized, resource-saving implementation of biointerface studies” (project acronym: UniBioface)
- Fraunhofer cooperation: IAP, IFAM, ICT within Simpromat (BMBF-funded; funding code: FKZ 01IO1902): Further development of the in-house developed in-vitro test system with focus on the serial production of individual components and the application in the field of anti-viral testing.
- Fraunhofer Anti-Corona research project with the topic “Effective and robust biocompatibility testing of 3D printed components for the prevention and therapy of COVID-19” (project acronym: BioKomp)