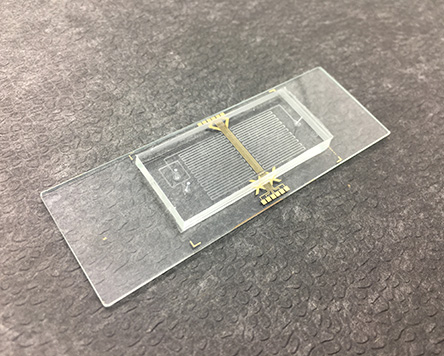
Fraunhofer IKTS supports these challenges as a development partner and service provider with high-performance materials, the latest measurement and diagnostic methods and innovative test environments for investigating material and cell interaction. Due to many years of experience in medical device development and validation as well as an excellent infrastructure, we can help manufacturers and users to qualify medical devices and technologies more quickly for application.
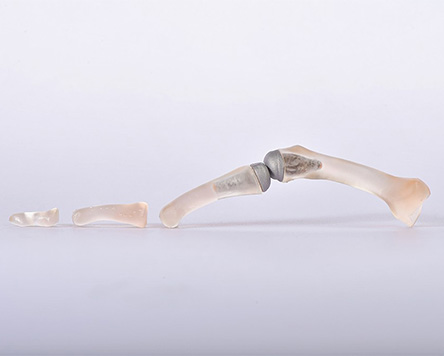
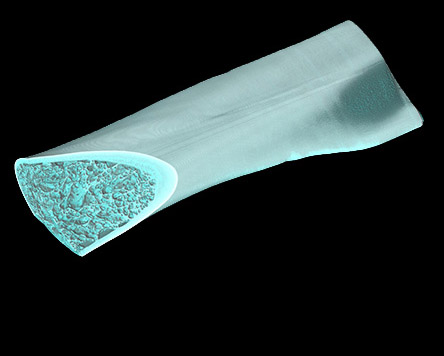
Using innovative high-performance materials such as ceramics, polymers and metals, the durability, biocompatibility and mechanical parameters of medical devices on and in the body can be continuously improved. As Europe's largest ceramics research institute, Fraunhofer IKTS has expertise along the entire value chain of (bio)ceramic and glass-ceramic materials for dental and implant applications. The researchers provide support in material synthesis and customer-specific material developments based on commercially available raw materials such as aluminum oxide, zirconium dioxide and non-oxide ceramics.
The experience gained is also used for polymer, metal and composite materials in order to achieve better product properties through material selection, material-specific shaping and functionalization. Interdisciplinary collaborations enable us to think up completely new medical products such as sensor-supported implants or integrated actuators and validate them for patients.
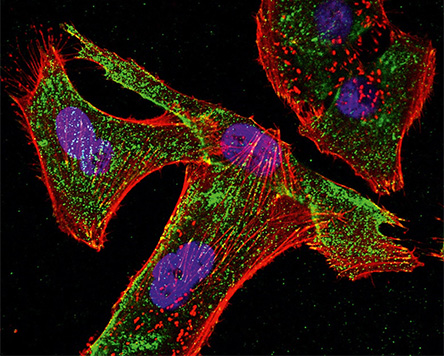
For the use of new components, substances or devices on and in the human body, Fraunhofer IKTS investigates the interactions between biology and material with research partners such as the Fraunhofer IZI. Through the collaboration of physicists, biologists and engineers on the one hand and standardized or specially developed test scenarios, measurement systems or analysis methods for in-vitro use on the other, we can close existing gaps in product development and evaluation. If necessary, these innovative diagnostic, measurement and monitoring procedures can also be accredited.