
Water-saving production of soda using electrochemical membrane processes
Current research


Soda and sodium bicarbonate are among the indispensable inorganic basic chemicals. They are used in many areas of daily life (detergents, food) as well as in numerous industrial sectors (e.g. glass and paper production). In Germany, more than 1.2 million tons of these chemicals were recently produced annually; the figure worldwide is around 35 million tons. It is produced either on the basis of naturally occurring trona (USA) or using the Solvay process on the basis of the raw materials brine, coke and limestone (I).
(I) 2 NaCl + CaCO3 → Na2CO3 + CaCl2
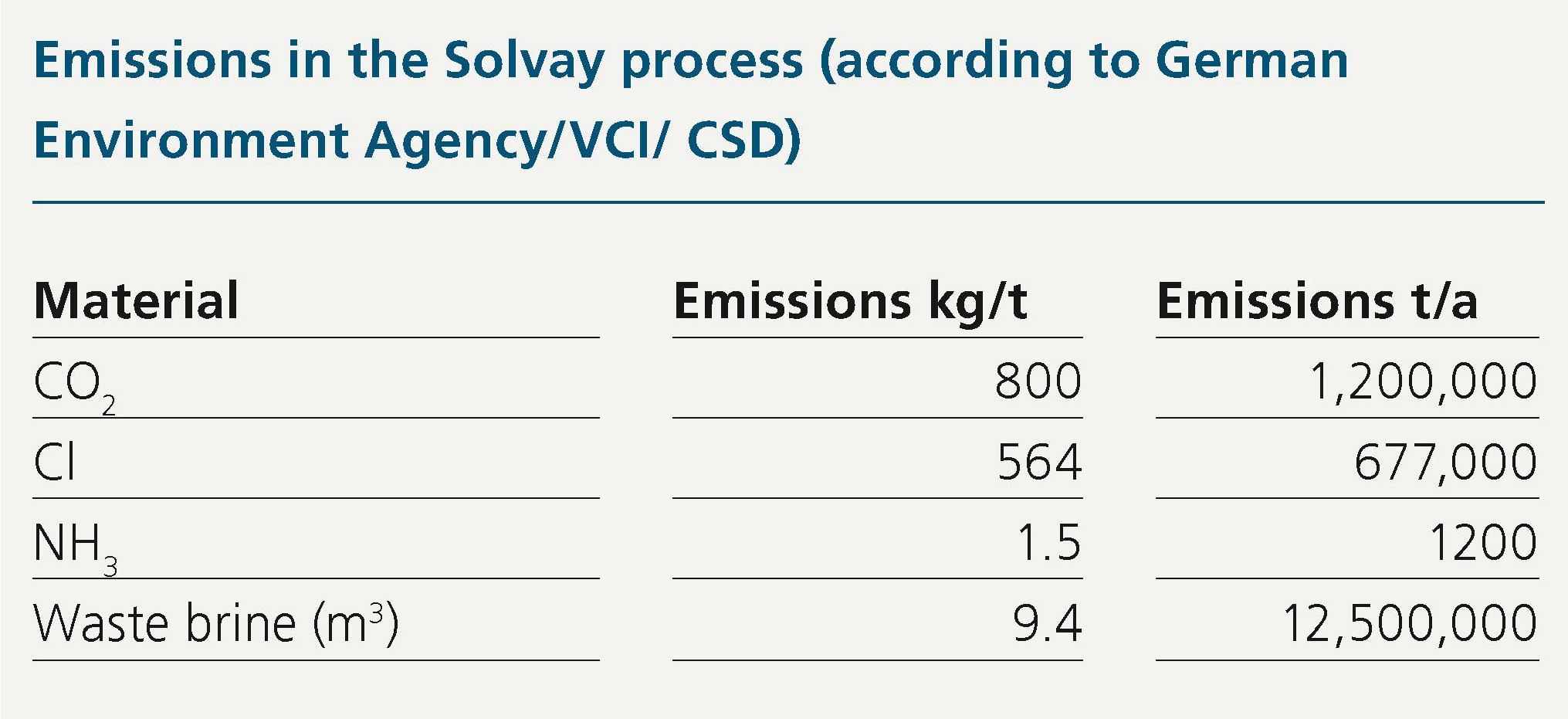
Ammonia is also required in the Solvay process as a carrier for chloride (Cl) and hydrogen carbonate (HCO3 ). Table 1 shows the raw material requirements and emissions.
At least as important as the high CO2 emissions in this context are the large quantities of high-salinity NaCl/CaCl2 waste brines that are produced and discharged to the next river. These lead to salinization of the receiving waters, with numerous other negative consequences such as fish mortality at low water levels and increased temperatures.
An alternative process for the production of soda is therefore being developed and tested as part of the BMWK-funded joint project “GreenSoda” (funding code 03EE5121A) with the participation of Stassfurt-based CIECH Soda Deutschland GmbH (CSD) and others. The effort centers around an electrochemical process route with bipolar electrodialysis. Figure 1 shows a laboratory test rig as a four-circuit system. The electrochemical process route enables the splitting of salt solutions into the corresponding acids and bases – in the case of NaCl brines, the splitting into HCl and NaOH according to (II). NaOH is subsequently carbonized with CO2 (III).
(II) 2 NaCl + 2 H2O → 2 NaOH + 2 HCl
(III) 2 NaOH + CO2 → Na2CO3 + H2O
The CO2 is obtained from combustion gases but also from fermentation processes (biogas production). At present, it is possible to produce an approx. 20 % soda solution based on raw brine from Stassfurt under laboratory conditions.
Neither limestone nor ammonia is required for this production route. This also means that CaCl2 waste brines are no longer produced. Now, the process even represents a CO2 sink. Fraunhofer IKTS has applied for a patent for this process together with CIECH Soda Deutschland.
The next step will be testing on a pilot-plant scale using purified CO2 from combustion processes and investigations on adapted thermal treatment processes. The latter are taken over by another network partner.
Supported by
