
Dynamics of alkaline water electrolysis
Current research

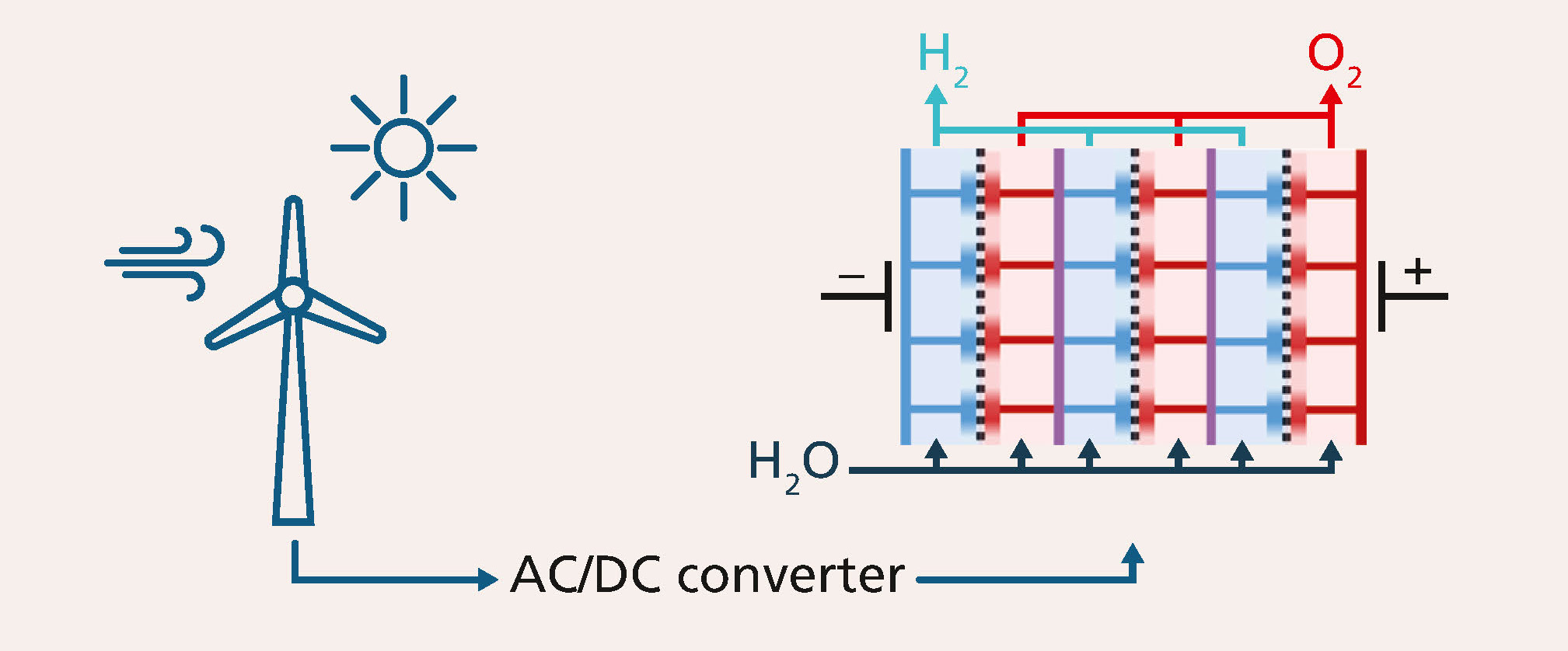
Due to the expansion of fluctuating renewable energies and an increasing number of “prosumers” (both producers and consumers), the energy grid’s more traditional, stationary mode of operation has reached its limits. The need for dynamic electro-chemical processes for energy storage is growing. Consequently, water electrolysis, a promising green energy storage solution, has to be sufficiently dynamic – a real challenge for industry. In this context, various processes occupying different (continuous and non-continuous) time scales need to be looked at:
- Electrochemical transients in milliseconds
- Load changes of the electrolyzer in seconds
- Cold and warm starts in minutes to hours
- Degradation behavior measured in days to years
In industry and science, the prevalent assumption is that only PEM (proton exchange membrane) water electrolysis is suitable for volatile energy generation because of its more dynamic response. By contrast, alkaline electrolysis is often not considered in the design of electrolyzer plants, despite the fact that other indicators (e.g. gas purity, load change time) should give that technology an edge. Scientific investigations carried out by the WaTTh Hydrogen Application Center at the Arnstadt site of Fraunhofer IKTS have shown that alkaline water electrolysis can indeed keep up with the transients of volatile renewable energies. Using this knowledge, researchers are working on the development of an optimized stack for a dynamic mode of operation.
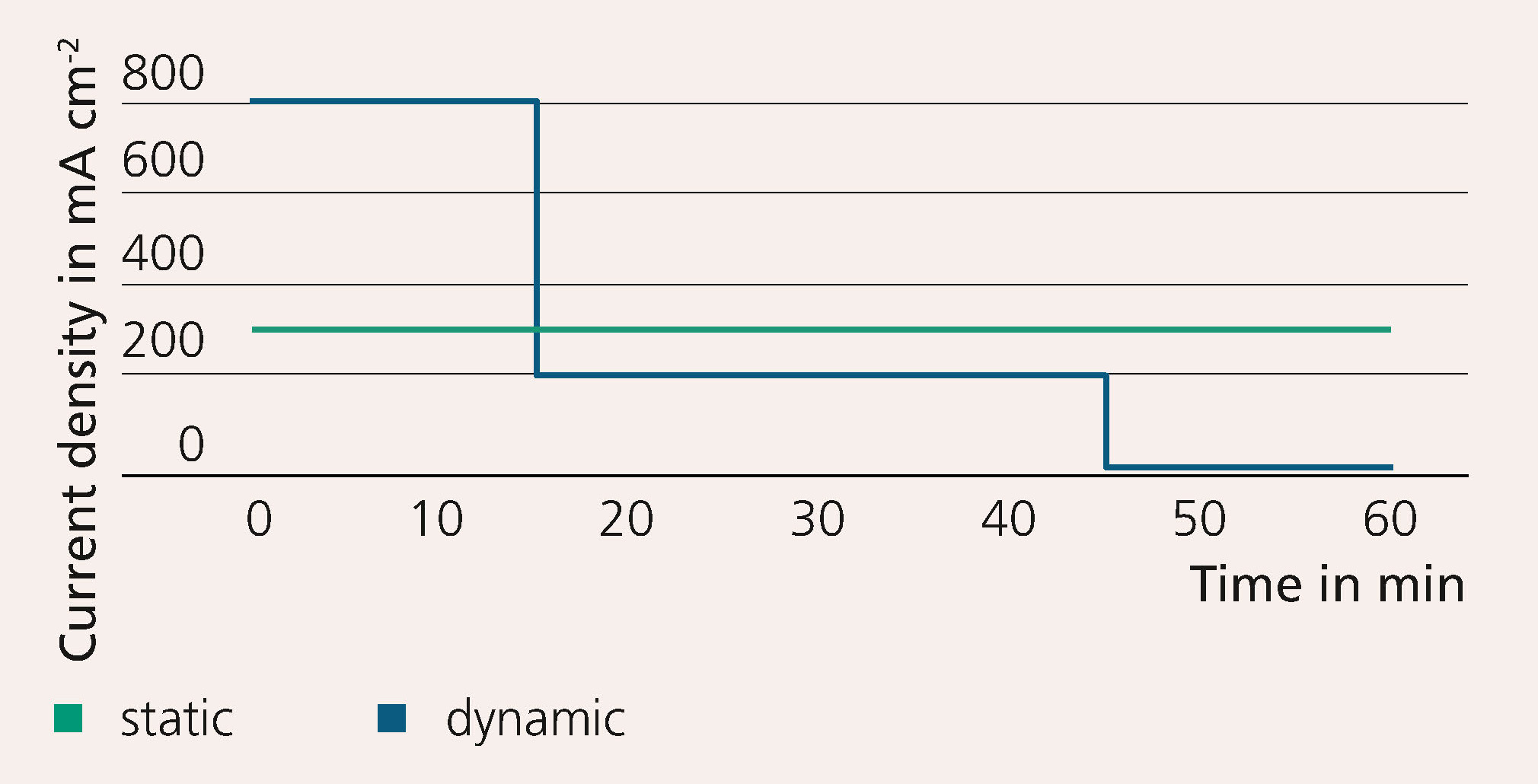
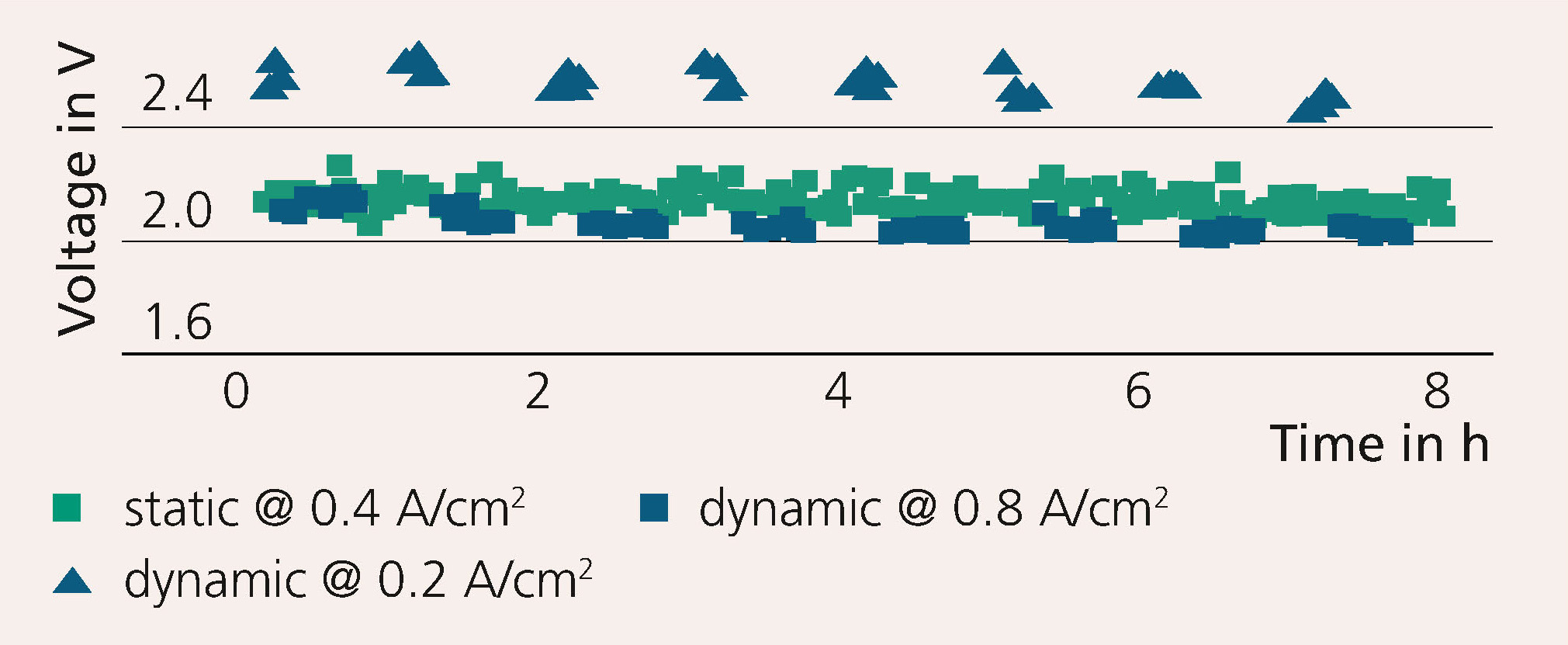
The research team developed an electrochemical test cell with an active area of 100 cm2 and an automated test stand for alkaline water electrolysis for scientific analysis. The test protocol shown in Figure 3 illustrates the cell’s resistance and voltage degradation.
The catalyst-free system showed strong efficiency losses of more than 0.4 A/cm2. Within eight cycles, no degradation was observed. However, investigations using impedance spectroscopy show that the resistance for the electron transfer reaction decreased. The resulting roughening of the surface indicates a larger number of reactive centers. These developed during dynamic processing, even though the number of electrons transferred was the same as for the static reference protocol. This indicates harsh conditions at the elec-trode surface, which can lead to delamination or other degradation phenomena. In future, the influence of other operating conditions, such as temperature, volumetric flow rate of electrolyte or bipolar plate geometry, towards dynamics in alkaline electrolysis will also be investigated. Another focus will be on the purity of the gases produced in the dynamic process. The ultimate goal is to develop a 100 kW stack design that is optimized for a dynamic mode of operation.
Supported by

